


Protein-based composite biomaterials
Proteins are self-assembling biopolymers that play central role in living organisms. All organs, including skin and eyes, are made from proteins, as well as muscle, hair and nails. Our digestive system, immune system, blood, vision and even regulation of genes need proteins to work as planned.
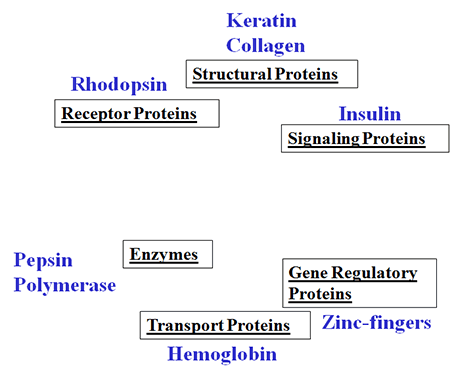
Protein-based materials are natural biomaterials that use proteins/peptides as building blocks and are employed as scaffolds in tissue engineering due to their advantage of biocompatibility, ability to signal fundamental cellular processes and a broad range of functional requirements that they can satisfy.

These materials are similar or identical to macromolecular substances recognized by biological environments and are relatively easily processed under variety of physical and colloidal states.
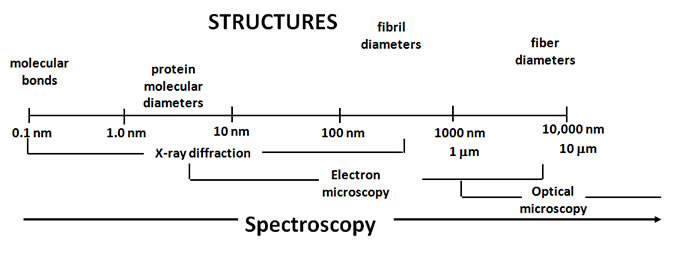
The design flexibilities in protein-based materials come from their naturally diverse and hierarchical levels of organization. The extensive arrays of structural elements are organized at length scales that range from Angstroms to microns and higher dimensions. The complexity of such organization creates a need for multi-scale approaches to integrate molecular, micro- and macro-molecular level of understanding of the protein self-assembly processes in order to pursue protein-based material design.
In the last several years, we worked with collagen-based materials (collagen was interfaced with co-polymers including hyaluronic acid (HA) and chondroitin sulfate that it interacts with in the extracellular matrix (ECM) of tissues, medical devices, etc.) organized in the form of hydrogels. We focused on the production of new knowledge, however, the strong fundamental foundation we established through our research, opens new directions and application opportunities.
Collagen
Collagen is a major structural protein that comprises up to 35% of the whole human body protein content. It is a main component and a source of strength of skin, tendons and ligaments. Blood vessels, corneas, cartilage, bones, intestines, inter-vertebral discs, dentin, the lining of muscle fibers and the heart are all rich in collagen. It holds everything together and helps to prevent the spread of disease-causing organisms, toxins and cancer. Modification to this protein’s structure has a significant impact to body’s health.
Collagen is believed to promote youthful hydrated skin, to reduce the appearance of wrinkles, to maintain skin elasticity and health. If there is no correctly formed collagen present, cancers develop, wounds do not heal properly, and the cuts on skin remain for a long time and get infected.
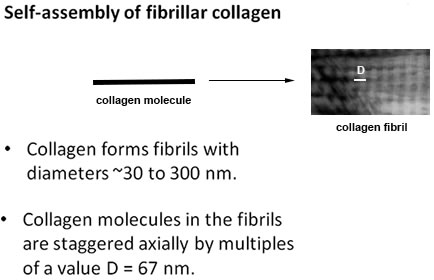
The knowledge of collagen formation currently exists in chemical and biological fields. In chemical and biological disciplines, however, the knowledge is limited to understanding the collagen molecules. The grand challenge is to design and make structures out of collagen molecules that resemble materials that humans are made of in terms of structural, chemical and physical properties. This challenge requires moving collagen and other protein research into the realm of Engineering. Overcoming this grand challenge will lead to being able to produce replacement of damaged body parts and to guide their repair. Knowing what collagen looks like at the tissue level will help people to understand the process of staying healthy, and to make the right decision about healthcare products and available treatments.
Collagen hydrogel materials - 3D biopolymer networks - proved to be ideal prototype systems to study collagen arrangements at all levels due to their size and assembly mechanics.
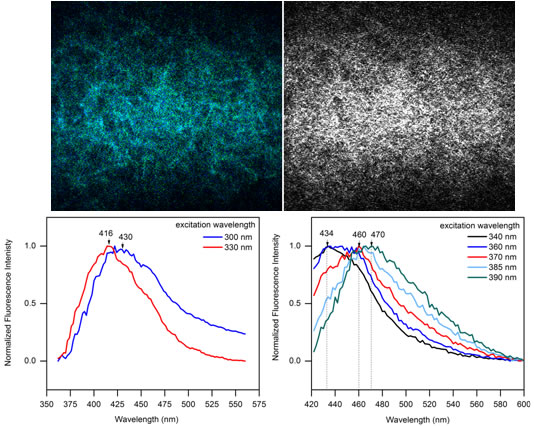
At UCR we worked with a number of cutting edge technologies to experimentally validate collagen protein structure in different materials to fill the existing knowledge gaps. For example, protein secondary structure was determined with circular dichroism spectroscopy, while transmission electron microscopy was used to visualize resulting fibrillar and/or filament nanostructure morphologies. Raman scattering based techniques provided additional molecular information in determination of conformation, density, stress-strain changes and other properties relevant to supramolecular order, modification of protein structures and characterization of water associated with the materials we made.
The non-invasive nonlinear optical microscopy imaging methods we developed in our laboratory at UCR enabled us - for the first time - to literally see how collagen proteins form and disassemble at the material level. Guided by spectroscopy (the study of the interactions between matter and electromagnetic radiation), the imaging technology produced the needed pictures without harming the specimens. This capability to produce high contrast pictures deep inside opaque collagen and other materials that humans are made of has a far-reaching potential to transform biological, biomedical, agricultural and other not yet known research disciplines. Rheology experiments allowed us to further correlate the protein structures we detected with the shear forces that could be applied to materials.
The experimental work of our UCR laboratory on collagen hydrogel materials was highlighted in the RSC Analytical Methods - a Royal Society of Chemistry (RSC) venue. RSC Analytical Methods 7, 1680-1690, 2015 (right) was also chosen as a Hot Article (http://blogs.rsc.org/ay/category/hot-article/). Our other works received similar recognitions.
 |
 |
Analytical Methods 3, 529-536 (2011) Describes the methodology and findings on multi-scale characterization of collagen hydrogels assembled under different physicochemical parameters. |
Analytical Methods 7, 1680-1690 (2015) Describes the structural dependency of collagen fibers on ion types
|
In the tissue engineering and regenerative medicine applications it is often necessary to use chemicals to make protein-based and other natural soft materials and hydrogels more mechanically stable and durable. Using natural (green chemistry) reactions in this context offers many new opportunities. We therefore were prompted to take a lead to begin developing the standards in characterizing the effects of different non-toxic stabilizing chemicals, including sugars, with application of optical techniques of multiphoton microscopy and Raman spectroscopy.
 |
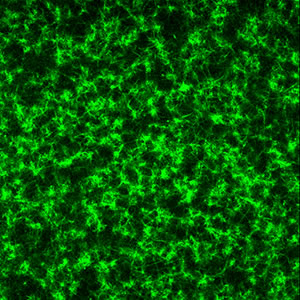
green: multiphoton fluorescence that developed upon cross-linking a collagen hydrogel with ribose ACS Analytical Chemistry 83, 200-206 (2011) ACS Applied Materials & Interfaces 3, 2579-2584 (2011) ACS Applied Materials and Interfaces 4, 261-267 (2012) |
Our studies of the modifying effect of sugars on collagen structure at the material level are also very relevant to ageing and diabetes research.
Eventually our work may allow the recognition of early diabetic complications and effectiveness of treatments directly through patients’ skin, in their arteries, tissues and major organs using light provided by laser devices to obtain instantaneous, detailed assessments of their function and health (“optical biopsies”).
The research we performed on collagen materials studied the interaction of light with matter and created knowledge of what generates the signals used to make pictures of this protein. This was an important milestone towards achieving “optical biopsies” of the future that can be performed in real time.
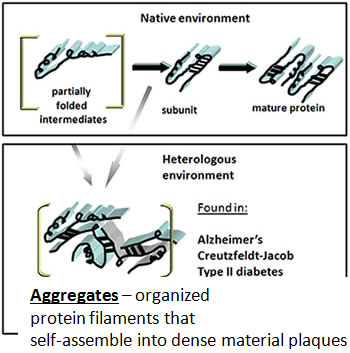
The cutting edge, interdisciplinary research that we perform on proteins may help someone to recover from or avoid developing Alzheimer's and similar disease, thought to be caused by incorrectly assembled proteins.
Journal of the American Chemical Society, 124, 14840-14841 (2002)
|
Copyright 2007 J.G.L. All rights reserved


Extra Links: